SEROMATRİX Semi-Quantitative Anti-SARS-CoV-2 (COVID-19) IgG ELISA KİT
Fast and easy detection of the coronavirus is extremely critical. Real-time polymerase chain reaction (RT-PCR) tests detect genetic material to perform coronavirus testing as a gold standard.
However, these tests have low rates of specificity and sensitivity and pose risks related to specimen collection and sample handling.
SEROMATRIX has developed a solution for researchers to minimize these risks by using IgG ELISA kits. SEROMATRIX has developed a semi- quantitative assay for IgG response to Anti-SARS-CoV-2 (COVID-19) antigen by using IgG ELISA kits.
Antibodies are produced gradually by the immune response system after infection.
IgG is an abundant immunoglobulin to be produced in response to an antigen and is maintained in the body after initial exposure for long term immune response.
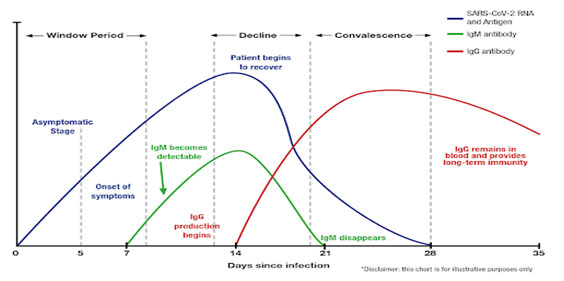
Seromatrix Anti-SARS-CoV-2 ELISA (IgG) is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection.
SEROMATRİX Anti-SARS-CoV-2 is an immunoassay for the in vitro detection of antibodies ( IgG) to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in human serum and plasma. Through a blood sample, the test can detect antibodies to the coronavirus, which could signal whether a person has been already infected and potentially developed immunity to the virus. This kit can provide highly accurate SARS-CoV-2 test results in approximately 50 minutes.
The determination of antibodies enables confirmation of recent or prior SARS-CoV-2 infection in patients with typical symptoms and in suspected cases.
It also contributes to monitoring and outbreak control.
Considering protective immunity, ELISA-based IgG serological test results can be used to determine the return time of individuals working in environments where they may be exposed to the virus. These Antibody detection tests are the only option that can be used to detect the infection, to determine the true extent of the pandemic, and to determine the rate of the case.
Due to the protection of the N protein sequence on the coronavirus and its strong immunogenicity, this protein is chosen as a diagnostic tool. Synthetic proteins representing different regions of the COVID-19 virus are plated onto plaques, while producing a kit to be used to determine the blood antibody level, blood antibody levels will be reported numerically.
The test will allow the monitoring of the infection as a result of numerical administration of the antibody level in the blood.
Thanks to the detection of positive antibody levels in the blood, the epidemiology of the disease is understood, and those who have undergone the infection without detection are identified and they are allowed to return to their jobs.
Individuals with negative antibody levels and at risk can be included in the group given priority in vaccination.

